Bryan L McLeod
Research continues to try and find why our crops and vegetables of today are so much lower nutrient wise than the same crops 30 years ago. The FDA in the US has reported that vegetables today contain 30% less nutrients than the did 30 years ago
Why you may ask? well there are two main reasons
1/ Plant breeders have been breeding to improve yields and reduced maturity time– great, the reduced time to maturity/harvest means crops grow quicker, are in the ground for a shorter time meaning your soil can be used more efficiently, reason you grow more crops. But in breeding for the so called “Better Plant” many beneficial factors have been overlooked and innocently bred out of the plant meaning that many of the nutrient advantages in a certain crop are no longer there.
Some non beneficial factors such as gluten has increased in our wheats, older species of wheat contained low levels of gluten compared to the newer species
2/ The introduction and higher use of nitrogen fertilisers.
Dilution: So what are we saying here? By increasing the speed at which a plant grows and reducing its time to maturity you effectively reduce the concentration of nutrients within that plant.
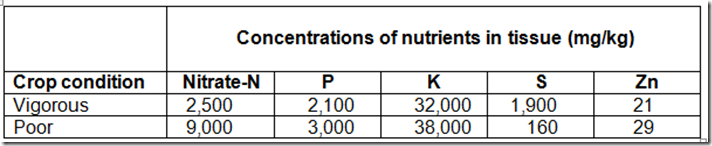
When a plant grows quickly it is taking up higher levels of nitrates from the soil, the plant cells expand and you have a greater amount of plant moisture but reduced nutrient content. This is demonstrated below. Also note the higher level of nitrates in the poor crop, in this case due to stress
Note the how the concentrations are lower in the vigorous compared to the poor – Supplied by SA Dept Ag
Now we want our crops to grow better than the poor but it does demonstrate how we can be nutrient deficient when consuming fast growing leafy vegetables.
This is a serious issue and to date seems to be un recognised in our human feeding plan but firstly we need to understand that we can easily change and correct this problem to our betterment, infact we have the knowledge and in my person experience we can correct and increase the nutrient content of our vegetables very quickly eg 1 to 2 weeks with soluble foliar applications
Pastures:
In the animal grazing pasture world we have moved on to the next stage, science and research did produce a number of faster growing higher producing pastures but when grazing some of these pasture species animal production decreased, some were unpalatable and one species resulted in serious health issues when grazed. Over the last 10 or 15 years pasture species have been and are being developed for nutrition, animal mineral levels and performance are measured as well as pasture growth factors. So in animal feeding they are one step ahead of humans.
With livestock we have been through the fast growing low nutrient pasture/vege stage and we understand the associated nutrient problems in our stock.
A large problem still does exist when stock are grazing fast growing pasture and that is the nitrate and lower nutrient issue, nitrates will always be high and remain high until plant growth slows down. Nitrates in plants are natural, the level of nitrates present depends on many factors
1/ Soil nutrient balance
2/ Growth conditions
3/ Weather conditions, cool weather plant growth slows = higher nutrient levels, warm weather = faster growth = lower plant nutrients, lack of sunshine = slower conversion of nitrates to amino acids, good sunshine = faster conversion of nitrates to amino acids
4/ Plant species: Some plant species are naturally higher in nutrients, eg Timothy, cocksfoot, prairie grass and clovers are naturally higher than rye grasses in their nutrient level. Pasture herbs like Chicory and plantain are higher again
Past information:
There is a lot of past information about experiences with nutrient dilution in pastures and resultant effects on livestock
Brown Trotter, a past sheep farmer from Fairlie in the South Island of New Zealand whom I had the pleasure of spending a day with back in the 80’s, wrote about his experiences in his book “Rape of Our Heritage” . When in the 1930’s as he introduced lime and fertiliser to his soils and increased his pasture growth and was able to significantly increase his carrying capacity. But he saw a significant down turn in individual stock health and performance. He noted a large number of tail end stock, stock that were just not doing so well something he hadn’t experienced before regular fertiliser applications and increase in pasture growth. These experiences were noted by many famers and became the centre of research in the that period.
Once Brown treated his stock with copper and cobalt all were healthy. This is just a typical example of pasture nutrient dilution and its adverse effect on livestock grazing that pasture. Now this was long before urea was introduced.
Nitrogen: Once urea was introduced and its use became more accepted farmers again started to experience serious nutrient issues in their stock plus increased metabolic problems, all again due to nutrient dilution in their pasture as a result of increased pasture growth.
DAP: When DAP fertiliser was recommended for use on New Zealand hill country sheep and beef properties farmers immediately experienced a decrease in stock performance and an increase in nutrient deficiencies. This was due to both the low sulphur input and the nitrogen effect on diluting pasture nutrient levels. Today if one was to suggest to a farmer to apply DAP to their pasture you would almost certainly be politely shown the gate
Supplements
Today in New Zealand and many other countries where stock are grazed on pastures, farmers need to and will address nutrient deficiencies with supplements, again we need to addressing this issue with humans. Not that we are grazing pastures but we are consuming vegetables that are grown quickly and they do contain higher levels of nitrates and do to plant breeders they are naturally lower in essential nutrients
Norfolk Island:
My son in law, James Partridge, on Norfolk Island told me how that the cattle grazing pasture along the roads needed to be treated with copper twice a year, but those grazing in areas where they had access to bush didn’t require the same copper treatment. This is an illustration of cattle being limited to pasture as against those having access to a larger range of plants
South Australia: A farmer related to me that before his father applied superphosphate to his soil his wheat yield was 1 bag/acre, after he applied superphosphate he achieved 8 bags/acre. (12 bags wheat/ton). We need this significant increase in our food production but we also need to maintain quality which is easier than you might think.
These photos show the extent of mineral deficiencies in Chinese crops that can be attributed to increased growth due to nitrogenous fertilisers being applied, but we must realise we need to achieved increased production, and we also need to recognise that we are getting nutrient dilution at the same time. Once we recognise this we can then use foliar fertilisers to correct the deficiencies created and still produce high quality nutritionally sound crops.
Zinc deficiency – note that the heads are not filled to the bottom
Copper Deficiency – the long bushy appearance
Boron Deficiency – rippling on the edge of the leaf – Cracking and uneven ripening in tomato’s
Potassium deficiency – tip die back garlic
Dilution of plant nutrients is a serious problem today and needs to be addressed, it is not a difficult problem to solve, we just need to do it by
1/ Balancing the soil minerals via a good soil analysis and fertility program
2/ Tissue test the plants
3/ Apply via foliar application the deficient elements to bring the plant to the optimum nutrient level
Foliar applications are an efficient way to finally correct and improve crop nutrition during the growing period
Recently I spoke with one of my old clients from WA, he told me how under my program he has build a reputation for his vegetable crops of quality, long shelf life and taste. But this year his normal fertiliser of sulphate of ammonia and sulphate of potash wasn’t available when he needed it so he used potassium nitrate and urea and immediately experienced a down turn in quality and shelf life.
China: Researchers in China over recent year have been trying to find out why vitamin B levels in wheat have decreased, once again dilution is the answer.